Would you like to make this site your homepage? It's fast and easy...
Yes, Please make this my home page!
|
The science behind electronics is the controlling electrons through resistors, capacitors, inductors, diodes, and many other devices that reduce, alter, or rectify the amount of electrons going through a conductive material.
| |
|
There is a basic law that was discovered by Charles A. de Coulomb that states that like charges repel and unlike charges attract. This is an important law because it explains why there is s nucleus and why there are shells and why the shells orbit the nucleus.
If you were divide a crystal of carbon time after time until it was no longer visible you would still be left with millions of carbon atoms. Keep dividing any you will eventually be left with one carbon element "carbon atom". If you were to divide it any more then you would be left with nothing more then protons,
|
electrons, and neutrons. At this level there is no distinct use for these particles since there can't be any solid objects or large forces of attraction.
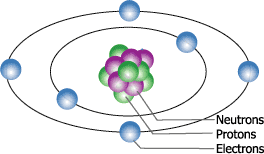
So lets stop at a single atom of carbon and examine it form there. As you look over the atom you will notice 3 distinct particles in two distinct formations. At the center of this model we see a cluster of protons and neutrons which form the nucleus. The nucleus is the heart of the atom because it dictates how many electrons the atom can carry, how strong the bond is with the electrons, and the majority of the atoms weight is with the nucleus, only about 3% of the weight is with the amount of electrons.
|
|
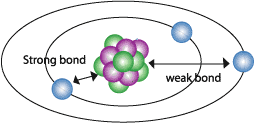
The amount of electrons an atom can carry is of great importance with electronics because if the atom can only hold 4 or fewer electrons then it is said to be an insulator which means that because the electrons are so close to the nucleus there is a great bond. This bond means that it does not accept or give electrons without a huge amount of energy. On the other hand if there is 7 or more electrons then the atom is said to be a good conductor because it has a third shell, which is farther away from the nucleus and has a weaker bond with the nucleus. This means that the atom can accept or give electrons with little or almost no resistant to electron flow.
The number of protons and neutrons also dictate what the atom is, it doesn't matter how many electrons an atom has because it will always have the same properties with respect with weight, color, and density.
|
|
The outer formation are know as shells and are where the electrons orbit the nucleus, the static charge between protons and electrons is so great that it is hard to understand what exactly static charge is. The electrons are orbiting the nucleus at speeds close to the speed of light and at this speed the electrons are in a constant struggle. If you have ever spun your arm around in circles you might notice a tingling sensation your fingers because of your blood rushing to your fingertips. This is the same with an atom, the electrons actually want to fly out into space but the static charge between the nucleus and electrons is great enough to keep them in perfect orbit. When the nucleus has the ability to accept another electron there is a rule that the electron must follow. Since like charges repel due to its static field that surrounds the particle, there is not enough room for more then 2 in a certain amount of space. The first field has only enough room for two electrons while the second field has enough room for four electrons. The more protons in the nucleus the more electrons the atom the bond with.
There is however two more rules that you need to know, the first is that the more shells there are the easier it is to it is to take away give electrons to another atom.
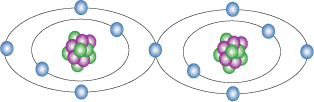 The second is if there are two atoms that have few protons come in contact with each other and one of them is deficient in electrons then the two atoms will share an electrons which means that both atoms are bonded together. This phenomenon is known as a covalent bonding and as a result this is why compounds exist.
The second is if there are two atoms that have few protons come in contact with each other and one of them is deficient in electrons then the two atoms will share an electrons which means that both atoms are bonded together. This phenomenon is known as a covalent bonding and as a result this is why compounds exist.
|
|
Current flow is the direction of electrons from an atom that has an abundance of electrons to an atom that has a deficiency of electrons. The atom with the abundance of electrons is said to have a negative charge because it contains more negatively charged electrons.
The atom with the deficiency of electrons is said to have a positive charge because it has more positively charged protons in the nucleus.
So with that you can come to the conclusion that electricity flows from negative to positive.
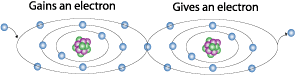
I wanted to add this paragraph because there is some confusion out there because Thomas Edison brought to the public that electricity flows from positive to negative, this layout is still used in many books today and is known as conventional current.
If you were to look at a diode schematic you would think that this is true, it is not but you can use conventional current, just be sure to always use one or the other never change your mind in the middle of a project.
|
|
Home |
Contact us |
Our History |
Link to us |
Relations
A Passion Production ©opyright 2000
